Researchers in the United States have produced novel lifeforms, which can be programmed to perform desired functions, using cells from the African clawed toad; Xenopus laevis. The aptly named Xenobot technology is proof of concept for increasingly sophisticated ‘biological robots’, with a range of applications in fields ranging from cancer treatment, to cleaning up pollutants in our oceans. The experiments focussed on the goal of producing lifeforms from frog skin and heart cell arrangements that could predictably move like a motor. Skin cells are passive and the heart cells, which naturally contract, would be an active component of this biological machine.
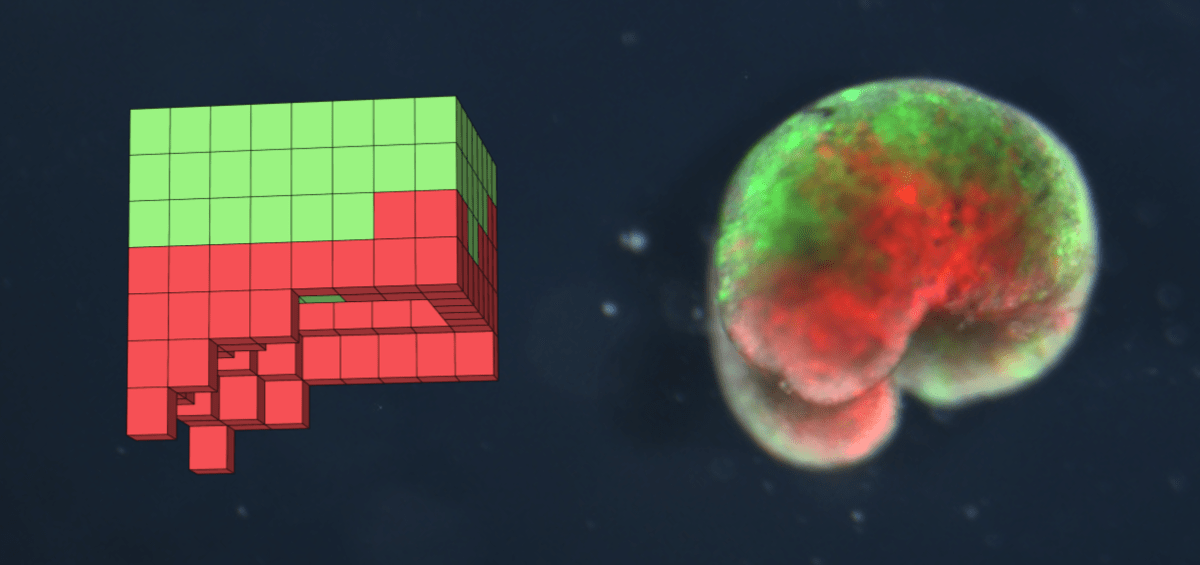
Technicians in Vermont used an evolutionary algorithm to first generate millions of different heart and skin cell combinations in 3D block arrangements. Then, each design was simulated in a physics-based virtual environment to judge how well they performed a desired function – locomotion. 99 rounds of selection were carried out, and after every iteration the designs with the lowest performance scores were removed and replaced with modified versions of the more effective designs. Eventually, this exercise produced numerous highly effective motor-like Xenobot simulations. This process mimicked natural selection so well that traits present in earlier iterations of the process were no longer present by the end of the process – this process usually plays out over many millennia of evolution.
Next, the final designs were converted to real arrangements of living frog cells. Scientists reared embryos and painstakingly removed, and combined, pluripotent stem cells, capable of becoming important cell types, using surgery tools under microscope. Technicians stimulated these cells to transition epidermal (skin) cells and sculpted them into the desired shape for specific designs. Scientists injected separate frog embryos with mRNAs which encourage cardiac cell development by preventing the development of several other competing cell types. These presumptive cardiac cells were surgically removed and fused to the epidermal cells according to the 3D designs using tiny cauterisation equipment.
Of course, this process was not without hiccups; the computer simulations initially could not replicate real-life conditions well enough to produce Xenobots that worked as they were designed to. However, the computer software was gradually improved by a process of trial and error to the point where Xenobot behaviour was predictable, with some moving in straight lines and spirals.
Novel lifeforms like Xenobots could be produced from human stem cells to remove plaque from arteries, chisel off minute calcium deposits in joints, regenerate organs, and even diagnose and deliver drugs to cancerous tumours. Xenobots have been shown to heal when cut manually but also have a self-limiting lifespan of around a week, becoming an inert mass of dead cells that the body can easily remove. Besides being a robust and non-toxic medical tool, Xenobots could be deployed to collect and dispose of pollutants in water systems including microplastics, oil, algal blooms, and even radioactive contaminants. This technology potentially has a bright future but there are understandably well-founded ethical concerns about lifeforms containing nerves, veins, and arteries. Hopefully, governments will legislate appropriately against automation and dangerous Xenobot use, or even unethical abuse of the novel lifeforms themselves.
Original paper: Kriegman, S., Blackiston, D., Levin, M., and Bongard, J. 2020. A scalable pipeline for designing reconfigurable organisms. Proceedings of the National Academy of Sciences. 117(4), pp.1853– 1859.
By Pearce Curran
Header image from Wikipedia.